Elements & The Periodic Table - WTF is Chemistry?
It's pretty much the Bible of chemistry.
In the last blog we talked a little more about atoms and their nuclei (nucleus in its plural form). We now know that nuclei have protons and neutrons in them, and that the number of protons in the nucleus defines the element.
Here’s what I haven’t told you yet:
There is a term for “the number of protons in the nucleus of an atom”, and its called the atomic number. We use the symbol Z to represent the atomic number.
Now remember, elements are just atoms with a specific atomic number. They can not be broken down into smaller substances (atoms are as small as it gets).
This brings us the “bible” of chemistry: the periodic table of elements.
It’s basically a list of all the elements. There are many different “types” of periodic tables, and they vary with the amount of information they provide on the elements. Some tables are simple and contain only the symbol of the element and its atomic number, like the one you’re about to see. Others might tell you more about the element, like its molar mass, electronegativity or metallic character (We will eventually go over all of those).
As promised, this is what a periodic table looks like:
In the periodic table, all elements (each one represented by letter(s)) are sorted according to their atomic number from left to right and downwards. The atomic number can be found right above the letters. It starts with H (Hydrogen, Z=1), then moves across to He (Helium, Z=2), then downwards to the left to Li (Lithium, Z=3).
You might have noticed each part of the table is colored differently. This is simply for representation and provides no other value.
For now, I want you to ignore the green part of the periodic table.
Groups and Periods:
As you can tell, just like any other table, it is made up of rows and columns. The rows are called periods (I numbered them with roman numerals for clarity) and the columns groups. You might have noticed that the 10 middle groups, colored in blue, are not numbered. These are called transition metals. These are not numbered because, by convention, they don’t belong to the main 8 groups. Sometimes you’ll find periodic tables that number them, it really doesn’t matter.
We won’t be focusing on transition metals. You just have to know they exist. I’m sure you’ve already heard of some of them like copper (Cu), silver (Ag) and gold (Au). The only group I’d like you to know by its name is the 7th main group, the halogens. These would be F, Cl, Br,… etc.
I previously mentioned that the periodic table can contain more information on elements such as their metallic character. In general, elements in the same group have similar characteristics. I won’t go into details, but it’s important to know which elements contain metallic character.
Metallic elements & non-metallic elements:
On the left side, the first 2 main groups are metals, except for hydrogen (It’s a non-metal gas. I know, why is it there then?). The transition metals in the middle are, well, metals.
It starts getting trickier on the right side of the table:
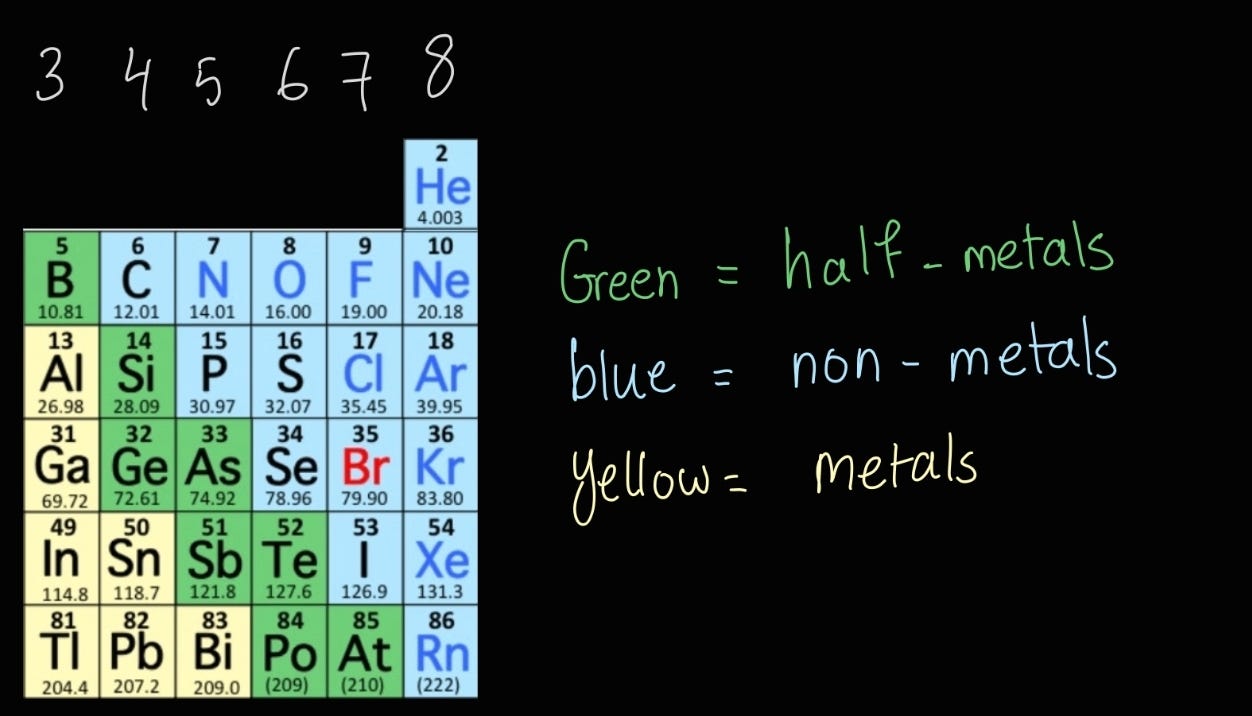
The further you go to the right side, the more non-metals you find. Some elements, mostly in group 3, are metals. Between metals and non-metals, you have “half-metals”, which show only partial metallic character.
Have a look at the image to help you visualize things a little better.
Di-atomic elements:
In nature, more noticeably in gases, some elements come in their di-atomic form. This simply means that instead of existing as a singular atom, the element likes to bond with another atom of the same element. They’re much more stable that way. For example, hydrogen gas exists in nature as H₂ (two hydrogen atoms chemically bonded). This would be a molecule, which we’ll go over in the next blog.
Remember the halogens I mentioned? (It’s the 7th main group) Most of them exist as diatomic molecules in nature. Other than the halogens and hydrogen, there’s oxygen (O₂) and nitrogen (N₂). You don’t have to memorize these. You simply need to know that they exist like that, so that you aren’t surprised when you come across them.
Alright, that’s enough for now.
I realize that this is a lot of information, so take your time getting to know everything. Try to slowly familiarize yourself with the different elements of the main group (which element each symbol represents).
See you soon.
Fun fact: Since elements are simply atoms with a specific atomic number, the periodic table was created before discovering all of the elements.






