When I first started studying chemistry, ions were the most difficult to grasp. I’ll spend a little more time explaining them, as I do now think that they’re much easier to understand than it seems at first.
What are ions?
Sometimes, atoms may gain or lose charges. The most important thing to understand is that they can only gain and lose charges through the transfer of negatively charged ELECTRONS. As you might already know, positively charged protons are located in the nucleus of the atom. They are unable to move around to charge or discharge other atoms.
When an atom gains an electron (by being strongly electronegative), it’ll gain a negative charge. When the atom loses the electron, it’ll “gain” a positive charge. This is what used to confuse me the most. Atoms never gain a positive charge, because protons are stationary; they do not move. Instead, they lose a negative charge, which equates to gaining a positive charge. It’s similar to subtracting by negative one:
-( -1 ) = +1
Please take your time to understand this concept, as it is immensely important.
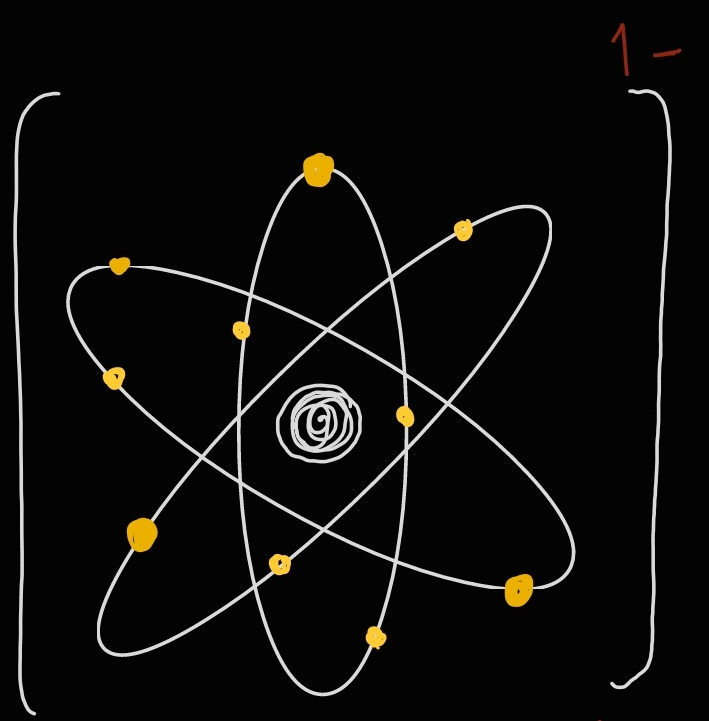
One thing I’d like to point you to in the image above is the fact that ions charges have a separate notation. We give an ion its charge by writing the number before the sign. For example:
Mg²⁺ NOT Mg⁺².
Hydrogen is an electron donor.
Remember when we were wondering in a previous blog why hydrogen is on the metals’ side in the periodic table of elements? Well, now I can tell you why. As you might recall, there are positively charged ions called cations (example: Na⁺), and negatively charged ions called anions (example: F⁻). All elements that form cations are actually metals, except hydrogen (that’s why they are put together), and all elements that form anions are non-metals.
The noble gases & the octet rule:
The reason why we are pretty much avoiding transition metals is because they don't always form ions with the same number of charges. They are more complicated, so we’ll carry on taking the easy way out and ignoring them. On the other hand, main groups do follow a general trend: the octet rule.
First of all, the 8th group, known as the noble gases, are extremely stable. They are so stable, that they don't want to gain or lose any electron, ever. They hate change and reactions. This is the goal of every other atom, to become as stable as one of the noble gases.
How do other atoms accomplish that? Well, by either stealing, sharing or giving away electrons.
Ionic bonding (the last intra-molecular force):
So, what do we know so far about ions? Here’s a quick recap:
We know they are formed by atoms that either rob or get robbed an electron. This causes both the thief atom and the victim atom to lose or gain a charge respectively. The atom with a greater electronegativity is usually the electron thief. Non-metal elements are usually the thief that steals electrons to become an anion, and metal elements are mostly the victims whose electrons get robbed (they become cations). Both atoms benefit from this, as they get a structure out of it, that makes them similar to their neighboring noble gas (which is very stable).
Well, what do we know about oppositely charged objects or particles? That’s right, they attract each other. This is basically how the ionic bond works. It’s also stronger than the covalent bond we covered last time!
To (hopefully) clear up any doubts, here’s a little sketch to help you visualize:
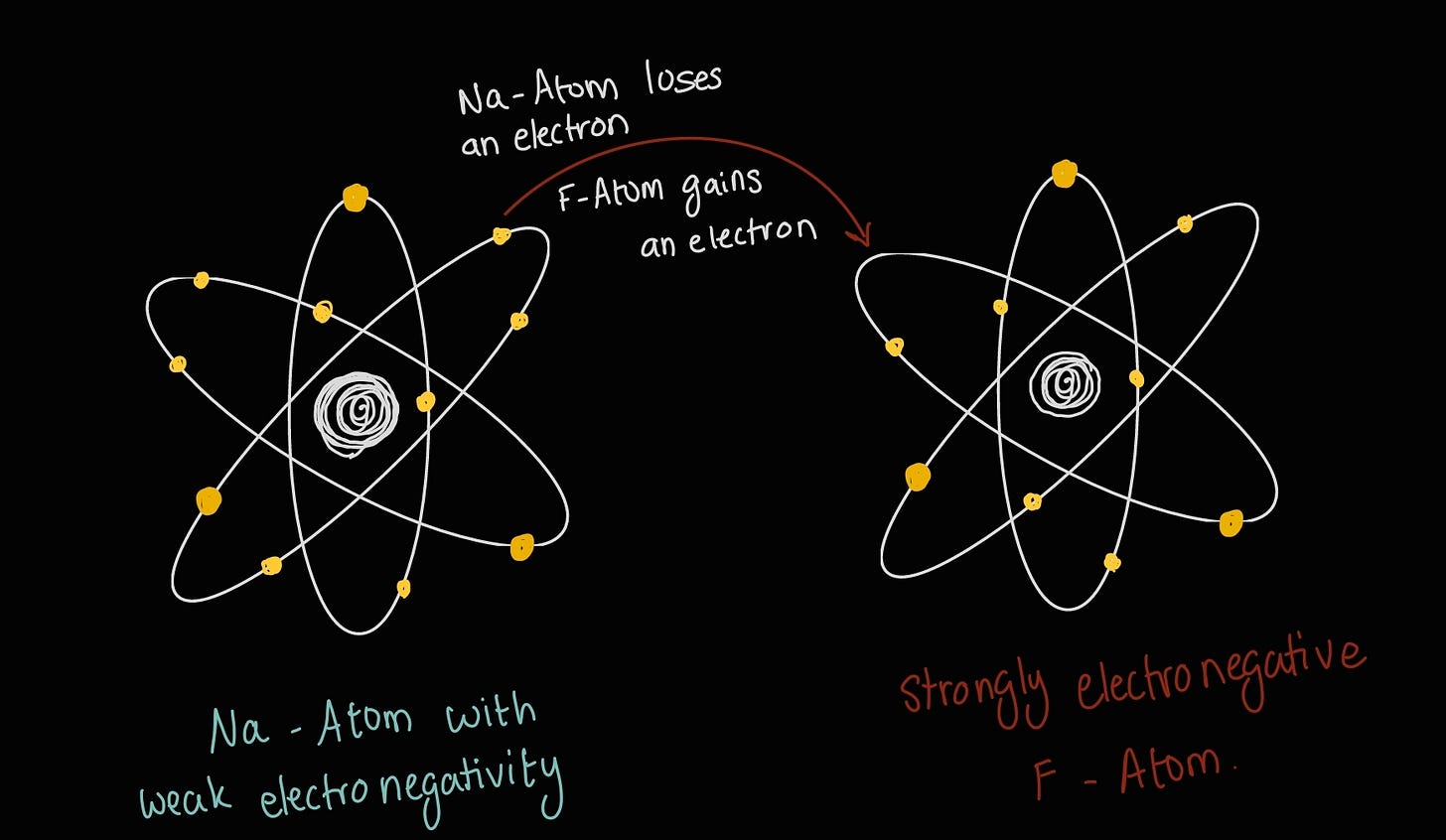
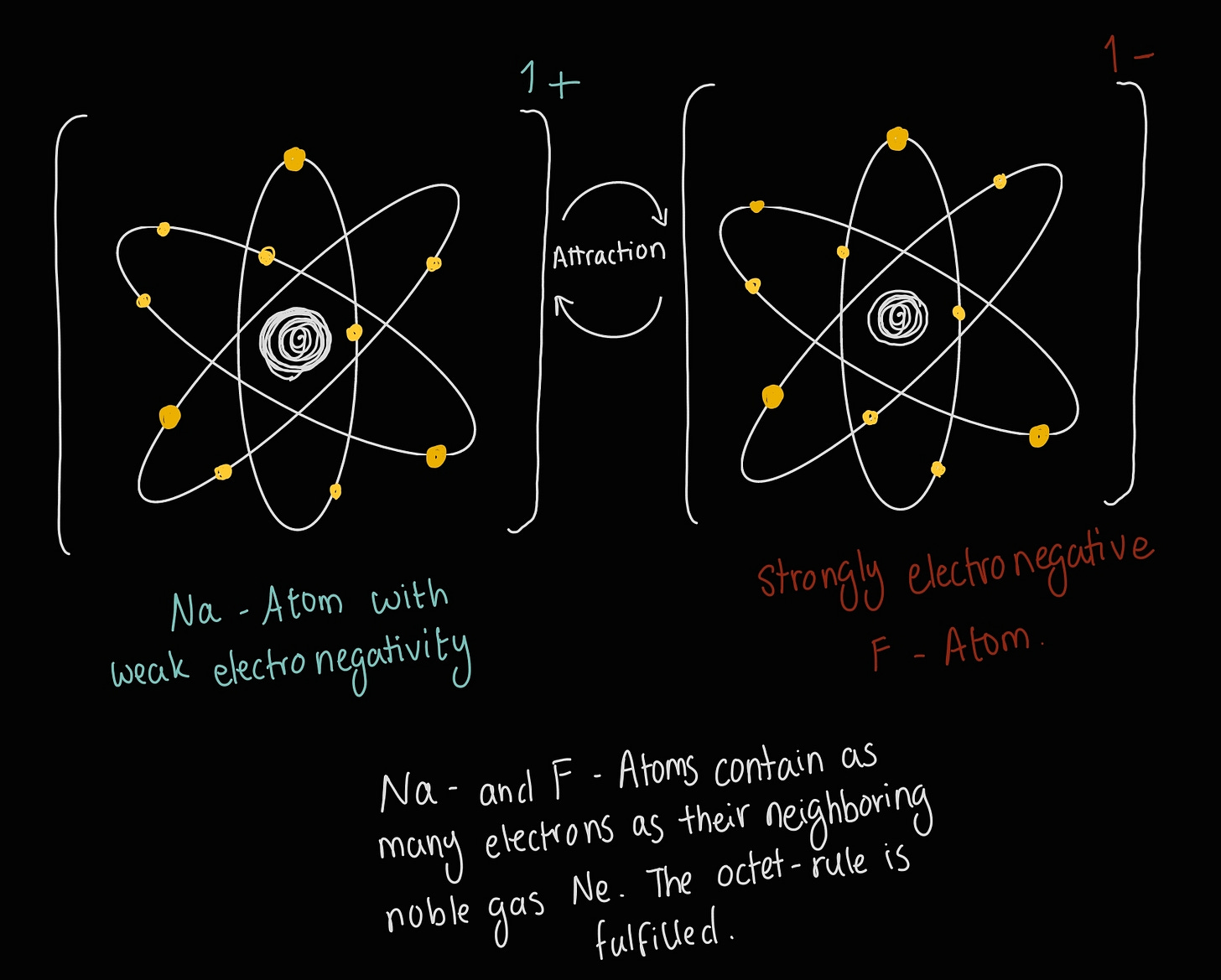
Fun fact: When you dissolve table salt (NaCl) in water, you are breaking this ionic bond that gives salt its crystal structure. You end up with Na⁺ cations and F⁻ anions in the water.
That pretty much covers it for ions and their bonding. In fact, that covers intramolecular bonding as a whole. Next time, we’ll jump head first into intermolecular bonding.
See you soon.





